- HOME
- Products & Services
- Sample Preparation Guide
Sample Preparation Guide
- Purification of DNA Templates
- Concentration Measurement
- Preparation of Sequencing Samples
- Preparation of Primer Plates
This guide provides information on how to prepare samples for our capillary sequencing service.
Concentration Measurement
Preparation of Sequencing Samples
Preparation of Primer Plates
Purification of DNA Templates
Contaminants in your sample can adversely affect the cycle sequencing reaction and result in low-quality data.
We recommend using commercially available purification kits for extracting and purifying DNA templates.
Specifically, PCR products must be purified as unreacted primers and dNTPs left after PCR can lead to poor sequencing results.
Optional Service
We also offer a purification service for PCR products.
If your samples are dissolved in buffers containing EDTA (like TE), EDTA can inhibit the cycle sequencing reaction. We recommend using sterilized water for preparing samples and primers.
Concentration Measurement
The amount of DNA template is critical for successful sequencing reactions.
For accurate quantification, we recommend using Qubit or agarose gel electrophoresis for measuring the concentration of sequencing samples.
Spectrophotometers may give inaccurate readings if residuals other than DNA are present.
For measuring concentration using agarose gel electrophoresis, use one of the following methods:
1. Estimate concentration by comparing the brightness with a known concentration molecular marker.
2. If no marker is available, prepare the sample so that a clear band is visible with 1 μL of sample applied.
Please provide an electrophoresis photo with sample application amounts and marker information (application amounts and concentrations). For visible bands, provide the original rather than a copy.
Example of Agarose Gel Electrophoresis
Plasmid
The following is the result of electrophoresing 1 μL of plasmid samples each.
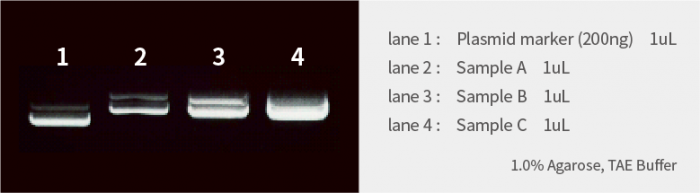
Based on the marker concentration of 200 ng/μL (lane 1), the concentrations of the samples are estimated as follows:
| Sample A(lane 2) | Approx. 150ng/uL |
| Sample B(lane 3) | Approx. 400ng/uL |
| Sample C(lane 4) | More than 400ng/uL |
However, Sample C appears saturated, so it is recommended to dilute and re-electrophorese for a more accurate estimation.
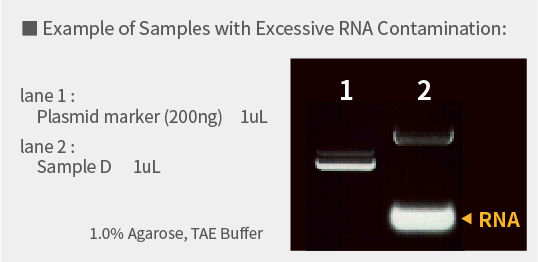
RNA Contamination
Excessive RNA contamination can inhibit the sequencing reaction. If RNA contamination is detected via agarose gel electrophoresis, remove RNA with RNase treatment.
PCR Products
The following is the result of electrophoresing 1 μL of PCR products each.
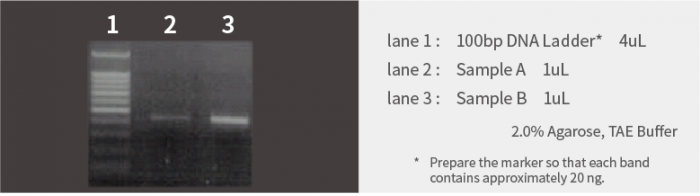
Based on the marker bands, the concentrations of the samples are estimated as follows:
| Sample A(lane 2) | Approx. 15ng/uL |
| Sample B(lane 3) | More than 60ng/uL |
Non-specific Products and Primer Residues
If multiple bands or primer residues are observed, perform gel extraction or remove the primers again. Components carried over from the PCR reaction can affect cycle sequencing.

Preparation of Sequencing Samples
Pre-mix
Mix the DNA template and primer (10 pmol) in the amounts specified in the table below and adjust to a total volume of 20 μL with sterilized water.
| Sample Type | Sample Size | Recommended Amount | ||||||
| Plasmid | – | 450 | – | 900 | ng | |||
| PCR Product | 100 | – | 200 | bp | 3 | – | 10 | ng |
| 200 | – | 500 | bp | 10 | – | 30 | ng | |
| 500 | – | 1,000 | bp | 15 | – | 60 | ng | |
| 1,000 | – | 2,000 | bp | 30 | – | 115 | ng | |
| > | 2,000 | bp | 60 | – | 140 | ng | ||
For a 200–500 bp PCR product
| DNA Template (PCR Product) | 20 ng |
| Primer | 10 pmol |
| Sterilized Water | up to 20 uL |
| Total | 20 uL |
In the pre-mix service, we use the sample prepared by the customer directly in a uniform amount for sequencing analysis. If the concentration deviates from the recommended range, the results may not be satisfactory.
One-pass / Full
Prepare samples and primers to the following concentrations and send more than the required amount.
- – Volume loss may occur due to evaporation, etc. when sending samples. Please be sure to send more than the required volume, even if the concentration is high.
- – If you wish to perform multiple analyses on the same sample, please send samples in a single tube in quantities corresponding to the number of analyses.
Primer Usage for One-pass 8-Strip / Plate
- – Up to 2 primers per 8-strip or plate. For 3+ primers, prepare a primer plate aligned with sample layout.
- – For 3+ primers, prepare a primer plate aligned with sample layout. For detailed instructions on preparing primer plates, please refer to Preparation of Primer Plates.
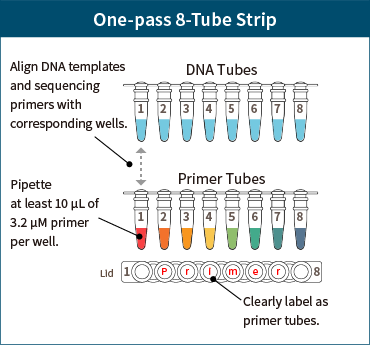
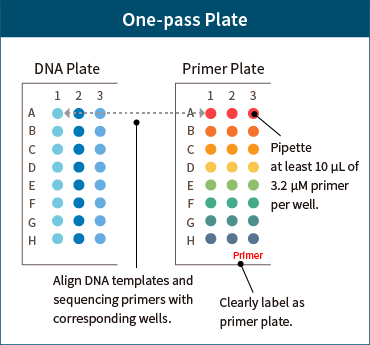
Preparation of Primer Plates
If using more than 3 primers in One-pass 8-tube strip or One-pass plate, prepare a primer plate corresponding to the DNA template.
Primer Plate
- – Prepare an 8-tube strip or plate separate from the DNA sample, aligning the primers to correspond to the DNA template.
- – Pipette at least 10 μL of 3.2 μM primer per well.
- – Clearly label the primer plate (tube) as “Primer”.