- HOME
- Products & Services
- DNA/RNA Large Scale Synthesis
DNA/RNA Large Scale Synthesis
DNA/RNA Large Scale Synthesis
Description
1. 1000mg / lot Production
Use of the dedicated machine for large-scale synthesis enables synthesis of 1000mg (2000mg for DNA under 30 bases) per one lot. All the products have their original lot numbers and are under the strict lot control.
2. Two types purification setting as default
Salt content is as important as purity in large-scale synthesis. This is the reason for setting RP-HPLC and desalting as a default purification method for this service.
Additionally, for RNA, tandem purification which is combined with IEX HPLC purification will be applied for the purpose of quality improvement. *1
3. High quality created by large scale synthesis
Our abundant experiences in smaller scale DNA and RNA synthesis as well as scale merit of one lot large scale synthesis achieve the products’ high quality. QC in the synthesis process and high purification technique ensure high purity of over 95% for both DNA and RNAt. *2
4. Manufacturing line compliant with GMP
Reliable products can be provided by synthesizing in the manufacturing line compliant with GMP (Good Manufacturing Practice). We will support your R&D with stable clean quality.
5. Strict quality control system
All synthesized DNA are subject to quality control by polyacrylamide gel electrophoresis. For the products under 50 bases, any small deletion will be found by LC-ESI-MS spectrometry analysis system. RP-HPLC purification analysis is also applied for the purpose of complete QC.*3
6. Advanced quality control
Such experience enables us to offer some optional services such as purity analysis data attachment by RP-HPLC or IEX-HPLC. (Click here to the price for options)
Our technical service staffs are willing to respond to your various advices and requests.
*1 IEX HPLC purification is applied for RNA more than 10mg as a default.
*2 This purity is the analysis result of RP-HPLC area percent (UV260nm) under our conditions, for normal DNA and RNA under 30 bases. For DNA over 30 bases, it will be over 90%.
*3 RP-HPLC analysis result will be attached to the product as an optional service.
| Product Specification |
Yield guaranteed | up to 2000mg (1 lot, 30 bases or less)*1 |
| Synthesis length | up to 60 bases (up to 30 bases for phosphorothioated oligos) | |
|
Purification grade
|
Reverse-phase HPLC + desalted | |
|
Quality control
|
PAGE, LC-ESI-MS*2, Absorbance determination (yield) | |
|
Quality control
(optional) |
1. Reverse-phase HPLC analysis | |
| 2. Ion exchange HPLC analysis*3 | ||
| 3. Endotoxin measurement*4 |
*1 Orders for yield other than the above may be acceptable.
One lot large scale synthesis is available from 20mg.
It may take longer than the ordinary DNA synthesis service for 100mg or less orders. The maximum amount per lot is 1,000mg for the length of 30 bases or more.
*2 LC-ESI-MS is available for oligos up to 50 bases long.
*3 Ion exchange HPLC analysis is available for oligos up to 30 bases long.
*4 Guaranteed value is 5EU/mg or less. For modified oligos, please inquire.
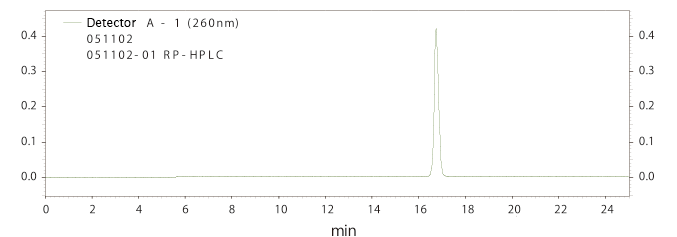
Reverse-phase HPLC analysis result for DNA of 24 bases long
Order Flow
Order |
Delivery date confirmation |
DNA/RNA synthesis |
Shipment |
|||
| → | → | → |
 Please order by Web or E-mail.
Please order by Web or E-mail. After receiving the order, confirmation of the product delivery will be sent by E-mail.
After receiving the order, confirmation of the product delivery will be sent by E-mail. Production and QC will be performed with our technique to produce high quality oligo DNA and RNA.
Production and QC will be performed with our technique to produce high quality oligo DNA and RNA. The products that are passed the final check will be shipped.
The products that are passed the final check will be shipped.
Ordering (Domestic)
Please inform us of the desired scale, chain length or quality control method (options). If the quotation was required, please E-mail to: dna@hssnet.co.jp. For domestic order,please download the order form from the following link, fill the necessary items, and send the form by E-mail.
- – For price list, click here.
- – For order sheet, click here
Cautions
Upon receipt of the order confirmation by E-mail, please check the ordered content (purification method or sequence) again.
GMP compliant large scale nucleic acid synthesis service
Gram scale RNA and DNA products are synthesized at GMP (Good Manufacturing Practice) compliant facility.
Based on our accumulated know-how of DNA synthesis, we will achieve our product concept, “high quality”, “rapid delivery” and “detailed service with customer first policy” also in our GMP compliant service.
We aim to be a partner for your research and development by our comprehensive products supply in the field of RNA research from screening to drug development. Products are available for the use in pharmacological tests, in vivo experiments and animal experiments, which will smoothly lead to the further stages such as drug development in the future.
Product Features
In RNA/DNA large-scale synthesis service, we will conduct consultations and carry out thorough quality control to meet customer needs.
– This service is available up to 60 bases for DNA synthesis and between 11 and 30 bases for RNA synthesis.
– For available synthesis amount, please inquire.
– Synthesis request for chimeric oligos and modified oligos (e.g. 2′-OMe, 2′-F) is also acceptable.
– Quality control during the synthesis process and advanced purification technology ensure purity of 95% or more for both DNA and RNA. *1
– HPLC and desalting are provided as a standard purification, and tandem purification combined with ion exchange HPLC purification is further performed for RNA products to improve quality. *2
– Purity analysis data by reverse-phase HPLC and ion exchange HPLC can be attached.
*1 Purity is the analysis result of reverse-phase HPLC area percentage (UV260nm) under our conditions for unmodified DNA/RNA of 30 bases or less. It becomes 90% for DNA of 30 bases or longer.
*2 Ion exchange HPLC is the standard purification for 10mg or more of RNA.
Standard analysis item
Purity measurement (HPLC analysis), molecular weight measurement (LC-ESI-MS)
Additional analysis items (optional)
– Endotoxin test, viable bacteria count test
– Quantitative test of impurities (heavy metals,residual solvent)
– Moisture content and salt concentration measurement
Manufacturing facility
This product is manufactured in a GMP compliant facility.

– Clothing, etc.
To prevent dust generation, dust-proof clothes are required and items to be brought into the laboratory are strictly limited.
– Air shower
Equipped with air shower to remove dust when entering the laboratory.
– Air conditioning, temperature and humidity control
Filtered clean air flows into the facility.Additionally, humidity and temperature are kept to be adequate for synthesis reactions.
– Lighting equipment
The lighting is designed to be embedded in the ceiling and is installed in a place isolated from the indoor environment, so there is no chance of dust falling.
– Easy-to-clean designed floors and walls
The flooring and walls are flat to control dust, and are designed to be easily cleaned.