- HOME
- Products & Services
- GMP Oligonucleotide APIs: Manufacturing & Quality Control
-
Oligonucleotide API CDMO
-
Custom DNA/RNA Systhesis
-
Next Generation Sequencing
- Next Generation Sequencing
-
Application Selection Guide
- Application Selection Guide
- Human Genome Sequencing
- Whole Genome Sequencing (Non-Human)
- Microbial genome sequencing
- Small scale sequencing (NGS Petit)
- GRAS-Di® Genotyping
- Repeat Motif Detection
- Library Prep for Challenging DNA Samples
- Gene Expression Analysis (Reference-Based)
- Isoform Sequencing (full-length mRNA-seq)
- De novo Transcriptome Sequencing
- Small RNA-Seq
- Microbial Community Analysis
- Shotgun Metagenomic Sequencing
- Metatranscriptome Sequencing
- ChIP-Seq
- CRISPR Screening
- Illumina Amplicon Sequencing
- PacBio Amplicon Sequencing
- Standard Pipeline Data Analysis
- Custom Data Analysis
- Gene Analysis from Pathological Specimens
- Sequencer Models and Sequencing Principles
- Sample Requirements
- How to Order
- Scientific Publications
-
Custom DNA Sequencing
-
Custom DNA Microarray
-
Protein Related Service
-
Laboratory Tools and Service
GMP Oligonucleotide APIs: Manufacturing & Quality Control
- Service Description
- GMP-compliant Oligonucleotide API Manufacturing / Process Development
- Quality Control / Analytical Method Development
Service Description
We provide oligonucleotide drug substances (APIs) for clinical and commercial use under GMP. (Launch in early spring 2026)
Beyond manufacturing planning, we flexibly design process development, scale-up, and analytical methods according to project objectives and development stages.
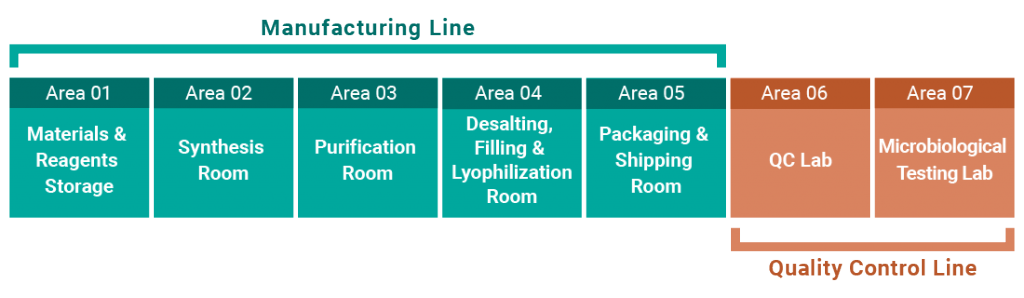
GMP-compliant Oligonucleotide API Manufacturing / Process Development
Manufacturing is conducted under a management system compliant with GMP and PIC/S.
Manufacturing methods, scales and analytical conditions are flexibly designed according to project objectives and development stages.
Quality Control / Analytical Method Development
We provide contract analytical services covering a wide range of quality tests required for nucleic acid therapeutics.
Analytical method development and synthesis of impurity reference materials (e.g., N-1/N+1 and depurinated species) are also available.
| No | Test Item | Equipment |
|---|---|---|
|
1
|
Appearance | — |
|
2
|
Purity (Assay) | HPLC |
|
3
|
pH | pH meter |
|
4
|
Identification Test (Molecular Weight) | Q-TOF LC/MS |
|
5
|
Identification Test (Sequence) | Q-TOF LC/MS |
|
6
|
Purity and Impurity Profile | Q-TOF LC/MS, HPLC |
|
7
|
Tm Value (Duplex) | UV-Vis Spectrophotometer with Tm Analysis System |
|
8
|
Water Content | Karl Fischer Moisture Titrator |
|
9
|
Sodium Content | ICP-MS |
|
10
|
Elemental Impurities | ICP-MS |
|
11
|
Residual Solvents | GC-MS |
|
12
|
Endotoxin | Toxinometer |
|
13
|
Total Microbial Count | Membrane Filtration System |