- HOME
- Products & Services
- dR-GalNAc
-
Oligonucleotide API CDMO
-
Custom DNA/RNA Synthesis
-
Next Generation Sequencing
- Next Generation Sequencing
-
Application Selection Guide
- Application Selection Guide
- Human Genome Sequencing
- Whole Genome Sequencing (Non-Human)
- Microbial genome sequencing
- Small scale sequencing (NGS Petit)
- GRAS-Di® Genotyping
- Repeat Motif Detection
- Library Prep for Challenging DNA Samples
- Gene Expression Analysis (Reference-Based)
- Isoform Sequencing (full-length mRNA-seq)
- De novo Transcriptome Sequencing
- Small RNA-Seq
- Microbial Community Analysis
- Shotgun Metagenomic Sequencing
- Metatranscriptome Sequencing
- ChIP-Seq
- CRISPR Screening
- Illumina Amplicon Sequencing
- PacBio Amplicon Sequencing
- Standard Pipeline Data Analysis
- Custom Data Analysis
- Gene Analysis from Pathological Specimens
- Sequencer Models and Sequencing Principles
- Sample Requirements
- How to Order
- Scientific Publications
-
Custom DNA Sequencing
-
Custom DNA Microarray
-
Protein Related Service
-
Laboratory Tools and Service
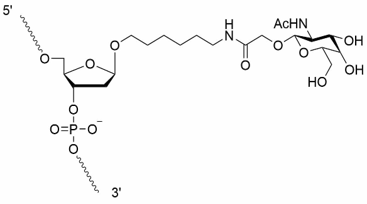
dR-GalNAc
| dR-GalNAc |
 |
This product is manufactured and sold for research use only under a joint license from LGC Genomics Ltd and MiNA Therapeutics Ltd.
For any clinical, diagnostic, therapeutic, or other commercial use, you must obtain an appropriate license directly from LGC Genomics Ltd or MiNA Therapeutics Ltd.
For details regarding the required licenses, please refer to the information below.
The GalNAc reagents [dR-GalNAc (beta) phosphoramidite and dR-GalNAc (alpha) CPG] are offered as oligonucleotide modifications exclusively under joint license from MiNA Therapeutics Limited and LGC Genomics Ltd., and for which patent protection is being sought through patent applications stemming from International (PCT) patent application publication number WO2021/032777A1.
GalNAc reagents are offered under the condition that the modified oligonucleotides be used for research use only and are prohibited from use in commercial applications (including, but not limited to, clinical, diagnostic, therapeutic or other applications) unless explicitly authorized by separate written agreement with LGC Genomics Ltd. Please enquire via licensing@lgcgroup.com.A license is required from MiNA Therapeutics Ltd. to incorporate GalNAc-dR (beta) phosphoramidite or GalNAc-dR (alpha) CPG into small activating RNA (saRNA) for up-regulation. Please inquire via info@minatx.com.
Back to "Products & Services"