- HOME
- Products & Services
- Scale-Up Synthesis (final yield: mg to g scale)
Scale-Up Synthesis (final yield: mg to g scale)
Pricing & Lead Time
Guaranteed Yield
Product Features
1. Final yield available at mg–g scale
We offer oligonucleotide synthesis across a wide range of scales, from screening applications to in vivo studies. Final yields are available from 0.5 mg up to multiple grams, and we propose the appropriate supply volume based on your purpose and development stage.
2. Gram-Scale / Batch Synthesis
By utilizing our dedicated high-throughput synthesis lines, we can achieve high-yield production of up to 2 g per batch (for DNA sequences up to 30 mer).
All products are assigned a lot number, ensuring consistent lot control and highly reproducible quality.
3. HPLC purification as standard
Reverse-phase HPLC purification is performed as standard to ensure quality suitable for in vivo studies.
Na+ salt exchange (ion-exchange purification) is also available upon request.
4. Support for modified oligonucleotides
We synthesize a wide range of modified oligonucleotides, including artificial nucleic acids (e.g., 2′-MOE, 2′-F, Locked Nucleic Acid) and modified bases.
Fluorescent dyes and various linkers can also be incorporated.
If you are interested in any modifications listed or require other custom modifications, please contact us.
5. High quality through large-scale production
Consistent high quality is achieved by combining our expertise in small-scale DNA and RNA synthesis with large-scale batch production. Using strict in-process controls and advanced purification methods, we deliver DNA and RNA with purities of ≥95%.*
- Purity is determined by reverse-phase HPLC area percentage (UV 260 nm) under our internal conditions for unmodified DNA and RNA up to 30 mer. Purity for DNA over 30 mer is typically ≥90%.
6. Clean Manufacturing Environment
Production is carried out in cleanrooms with DNase-, RNase-, and endotoxin-free consumables to ensure safe use in in vivo studies.
7. Strict Quality Control
All synthesized oligonucleotides undergo PAGE-based quality control.
For products up to 50 bases in length, LC-ESI-MS analysis is also conducted to detect even minor base deletions.
Our technical support staff are happy to assist with your requests.
Reverse-phase HPLC reports are available as an optional service, along with additional analyses listed below.
Quality Control (Optional Services) |
Items |
| 1. Reverse-phase HPLC analysis | |
| 2. Ion-exchange HPLC analysis (up to 30 mer) | |
| 3. Endotoxin Testing* |
- For natural oligonucleotides, the guaranteed endotoxin level is ≤5 EU/mg.
No guarantee is specified for modified oligonucleotides.
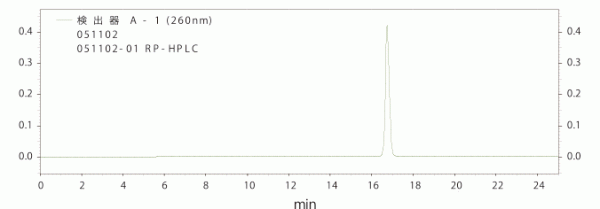 |
| Reverse-phase HPLC Chromatogram (24-mer DNA) |
Workflow - From Order to Delivery

- Depending on when we receive your order, synthesis may begin before we send the delivery schedule notice.
We will send an Order Confirmation Notice by e-mail, so please make sure to review the order details (such as purification method, sequence, etc.).
- Place your order via our website or by e-mail.
- After receiving your order, we will inform you of the estimated delivery schedule by e-mail.
- Synthesis of your oligonucleotide will begin, followed by a thorough quality inspection.
- Prior to shipment, we conduct final quality checks and deliver only products that have passed our quality standards.
Order Acceptance and Shipping Information
| Order cutoff time | Mon–Sat | E-mail / Web: by 16:00 | |
| Shipping | Mon–Sat | In operation | |
| Sun & Holidays | Closed | ||
| Delivery | Mon–Fri | For inquiries, please use our inquiry form. |