- HOME
- Products & Services
- GMP Oligonucleotide APIs: Manufacturing & Quality Control
-
Oligonucleotide API CDMO
-
Custom DNA/RNA Synthesis
-
Next Generation Sequencing
- Next Generation Sequencing
-
Application Selection Guide
- Application Selection Guide
- Human Genome Sequencing
- Whole Genome Sequencing (Non-Human)
- Microbial genome sequencing
- Small scale sequencing (NGS Petit)
- GRAS-Di® Genotyping
- Repeat Motif Detection
- Library Prep for Challenging DNA Samples
- Gene Expression Analysis (Reference-Based)
- Isoform Sequencing (full-length mRNA-seq)
- De novo Transcriptome Sequencing
- Small RNA-Seq
- Microbial Community Analysis
- Shotgun Metagenomic Sequencing
- Metatranscriptome Sequencing
- ChIP-Seq
- CRISPR Screening
- Illumina Amplicon Sequencing
- PacBio Amplicon Sequencing
- Standard Pipeline Data Analysis
- Custom Data Analysis
- Gene Analysis from Pathological Specimens
- Sequencer Models and Sequencing Principles
- Sample Requirements
- How to Order
- Scientific Publications
-
Custom DNA Sequencing
-
Custom DNA Microarray
-
Protein Related Service
-
Laboratory Tools and Service
GMP Oligonucleotide APIs: Manufacturing & Quality Control
- Service Description
- GMP-compliant Oligonucleotide API Manufacturing / Process Development
- Quality Control / Analytical Method Development
Service Description
We provide oligonucleotide drug substances (APIs) for clinical and commercial use under GMP.
Beyond manufacturing planning, we flexibly design process development, scale-up, and analytical methods according to project objectives and development stages.
GMP-compliant Oligonucleotide API Manufacturing / Process Development
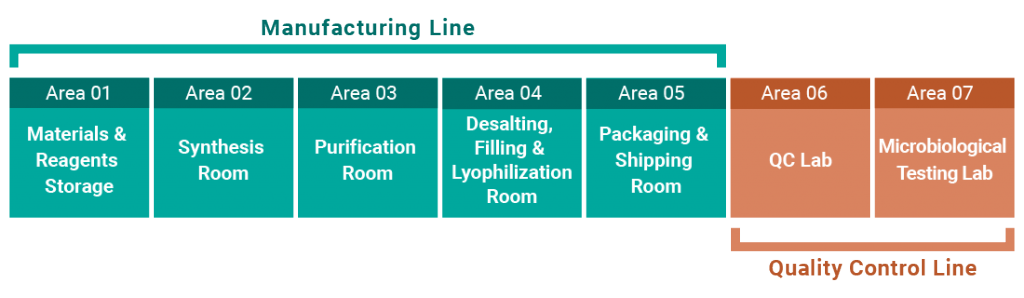
Manufacturing is conducted under a management system compliant with GMP and PIC/S.
Manufacturing methods, scales and analytical conditions are flexibly designed according to project objectives and development stages.
GMP Facility Layout (7 Areas)
Quality Control / Analytical Method Development
We provide contract analytical services covering a wide range of quality tests required for nucleic acid therapeutics.
Analytical method development and synthesis of impurity reference materials (e.g., N-1/N+1 and depurinated species) are also available.
List of Quality Tests
| No | Test Item | Equipment |
|---|---|---|
|
1
|
Appearance | — |
|
2
|
Purity (Assay) | HPLC |
|
3
|
pH | pH meter |
|
4
|
Identification Test (Molecular Weight) | Q-TOF LC/MS |
|
5
|
Identification Test (Sequence) | Q-TOF LC/MS |
|
6
|
Purity and Impurity Profile | Q-TOF LC/MS, HPLC |
|
7
|
Tm Value (Duplex) | UV-Vis Spectrophotometer with Tm Analysis System |
|
8
|
Water Content | Karl Fischer Moisture Titrator |
|
9
|
Sodium Content | ICP-MS |
|
10
|
Elemental Impurities | ICP-MS |
|
11
|
Residual Solvents | GC-MS |
|
12
|
Endotoxin | Toxinometer |
|
13
|
Total Microbial Count | Membrane Filtration System |