-
DNA・RNA・特殊合成
-
次世代シーケンス(NGS)
- 次世代シーケンス(NGS)解析
-
目的別:アプリケーション選択ガイド
- 目的別:アプリケーション選択ガイド
- ヒトゲノム解析
- ゲノムリシーケンス解析(ヒト以外)
- 微生物ゲノム配列決定
- 小スケール解析(NGS Petit)
- GRAS-Di®-ジェノタイピングシーケンス解析
- リピートモチーフ検索
- 一本鎖・微量・損傷 DNA対応ライブラリ調製
- 遺伝子発現解析(リファレンス配列のある生物)
- Iso-Seq 解析(full-length mRNA-seq)
- De novoトランスクリプトーム解析
- Small RNA-Seq解析
- 微生物群集解析
- ショットガンメタゲノム解析
- メタトランスクリプトーム解析
- ChIP-Seq解析
- CRISPRスクリーニング解析
- Illuminaアンプリコンシーケンス解析
- PacBioアンプリコンシーケンス解析
- 標準パイプライン解析
- カスタム解析
- 病理組織標本から遺伝子解析
- シーケンサー機種・原理
- サンプル必要量
- ご注文方法
- NGS論文実績
-
DNAシーケンス
-
マイクロアレイ
-
タンパク質関連
-
研究支援
-
関連製品
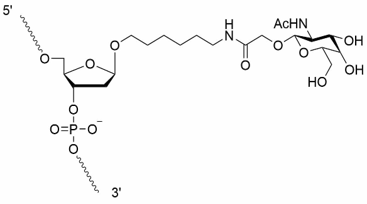
dR-GalNAc
GalNAc修飾は細胞(主に肝臓)への送達を高める効果が期待できます。天然の糖リン酸骨格のdR官能基を持ちます。
| dR-GalNAc |
 |
本製品はLGC Genomics LtdとMiNA Therapeutics Ltdの共同ライセンスの基に、研究使用目的で製造、販売しているものです。
ご購入いただいた製品を臨床、診断、治療等の商業使用される場合は、お客様にて別途、LGC Genomics Ltd、又はMiNA Therapeutics Ltdからライセンスを取得いただく必要があります。
ライセンスの詳細については以下をご確認ください。
The GalNAc reagents [dR-GalNAc (beta) phosphoramidite and dR-GalNAc (alpha) CPG] are offered as oligonucleotide modifications exclusively under joint license from MiNA Therapeutics Limited and LGC Genomics Ltd., and for which patent protection is being sought through patent applications stemming from International (PCT) patent application publication number WO2021/032777A1.
GalNAc reagents are offered under the condition that the modified oligonucleotides be used for research use only and are prohibited from use in commercial applications (including, but not limited to, clinical, diagnostic, therapeutic or other applications) unless explicitly authorized by separate written agreement with LGC Genomics Ltd. Please enquire via licensing@lgcgroup.com.A license is required from MiNA Therapeutics Ltd. to incorporate GalNAc-dR (beta) phosphoramidite or GalNAc-dR (alpha) CPG into small activating RNA (saRNA) for up-regulation. Please inquire via info@minatx.com.
サービス一覧へ戻る